|
INTRODUCTION
TO WATER TREATMENT & ABSTRACTION |
COMMON CHEMICAL MEASUREMENTS
pH TURBIDITY
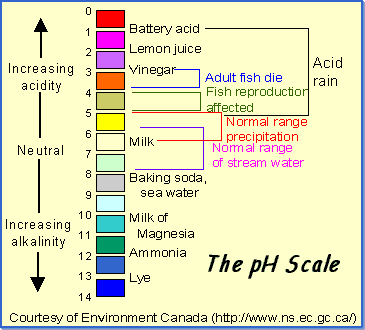
pH
pH
is a measure of how acidic/basic water is. The range goes from 0 - 14, with
7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater
than 7 indicates a base. pH is really a measure of the relative amount of free
hydrogen and hydroxyl ions in the water. Water that has more free hydrogen ions
is acidic, whereas water that has more free hydroxyl ions is basic. Since pH
can be affected by chemicals in the water, pH is an important indicator of water
that is changing chemically. pH is reported in "logarithmic units,"
like the Richter scale, which measures earthquakes. Each number represents a
10-fold change in the acidity/basicness of the water. Water with a pH of 5 is
ten times more acidic than water having a pH of six.
Turbidity
Turbidity is a measure of the cloudiness of water. It is measured
by passing a beam of light through the water and seeing how much is reflected
off particles in the water. Water cloudiness is caused by material, such as
dirt,iron and residue from leaves, that is suspended (floating) in the water.
Crystal-clear water has a very low turbidity. the materials that cause turbidity
in our drinking water either settle out or are filtered before the water leaves
our treatment works. Turbidity is measured in nephelometric turbidity units
(NTU).

