|
INTRODUCTION
TO WATER TREATMENT & ABSTRACTION |
DISINFECTION
A
brief history
Chlorination
Alternative methods of disinfection
The process animated
A brief
history
The first permanent Chlorination plants
were installed in England in 1905 in an attempt to reduce the outbreaks of Typhoid
& Cholera,
The whole of London's water supply was being chlorinated
by 1936.
The last major outbreak of Typhoid was in Croydon in 1937 this
was attributed to somebody carrying the bacteria & not from the water supply.
The introduction of chlorination plants also helped reduce the occurrences
of other bacteriological & virus outbreaks such as Dysentery, Meningitis,
& Polio.
Chlorination
Chlorine is used primarily to kill micro
organisms, the Volume of chlorine required varies greatly depending on the source
& quality of the water to be treated. For example water from a river will
require much higher dose rates than water taken from a good quality borehole
source.
Another factor to consider is the network that the final water
is being distributed, to for example in a large town where the water will be
used fairly quickly the chlorine residual in the water will probably not need
to be as high when compared to a rural area where the water will need to be
distributed over a large area & consumption will not be so great.
The amount of chlorine required is called DEMAND & is generally measured in mg/l
(milligrams per litre) or ppm (parts per million)
There are three main types of
chlorination these are:-
Simple chlorination
Breakpoint chlorination
Super chlorination
Simple chlorination will normally be used on sites where water quality is very good
& the bacteria content is very low.
This is normally limited to good
quality borehole sources or later on in a treatment process when rechlorination
is required.
Breakpoint chlorination occurs when the chlorine in the water reacts with other chemicals
already present in the water for example, ammonia will reduce the effectiveness
of chlorine, after the chlorine has neutralised the ammonia the chlorine that
remains is used for the disinfection process. Obviously any change to water
quality can have a large effect on the chlorine demand & it is important
that the contact time is maintained to ensure the process is complete before
water is pumped to service reservoirs.
The best method for controlling
this process is with a process loop that constantly
monitors the chlorine residual present at various points in the treatment process.
Super chlorination is most commonly used when the water being treated will have
a very high bacteria content generally this will be river sources or where some
form of pollution has occurred. Dose rates in treatment works requiring super
chlorination can be very high.
Alternatives methods of disinfection
Ozone
is probably the most effective method of disinfection & is particularly
good at removing cryptospuridium.
While ozone is good for disinfection it
has disadvantages primarily cost & safety. Another disadvantage is that
there will be no chlorine residual present after the water has been treated
so chlorine will need to be added before water can be pumped to the consumer.
Ultraviolet Light
is a potent disinfectant, A water flow is passed over a stream of light emitted
from a UV lamp. The radiation produced from the light is absorbed by any micro
organisms in the water which changes their basic structure & leads to them
being killed.
The main disadvantage of ultraviolet light is that there
will be no chlorine residual present after the water has been treated so chlorine
will need to be added before water can be pumped to the consumer.
The
process animated
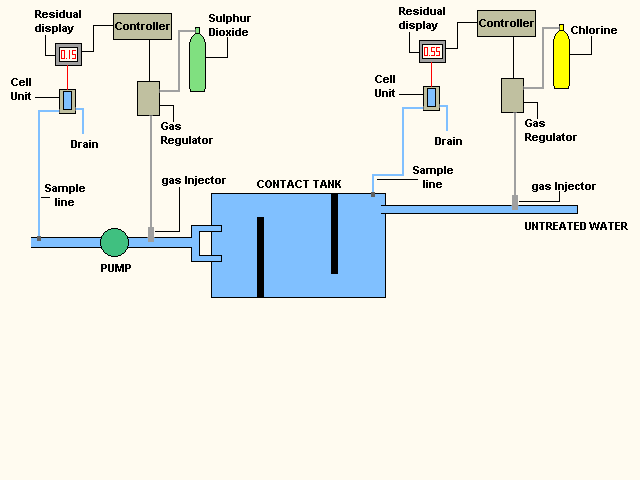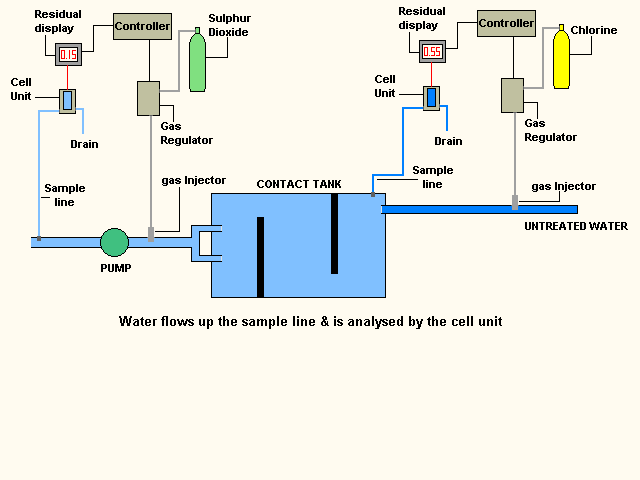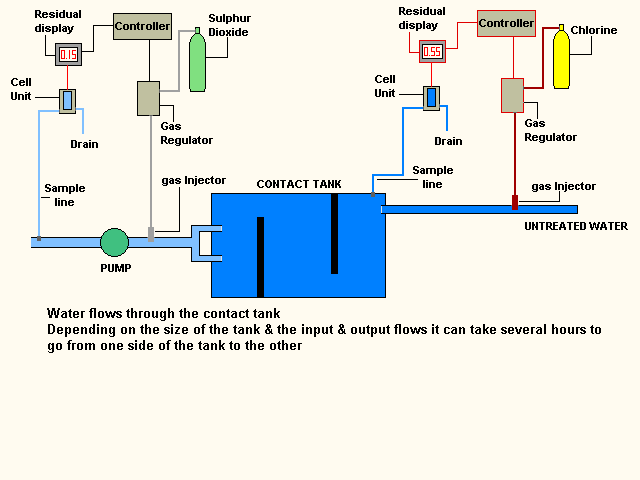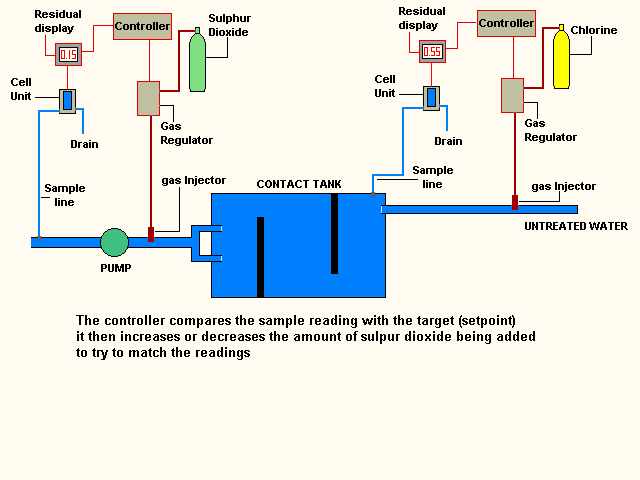
The animation below shows two process control loops
The first controls the amount of chlorine or sodium hypochlorite
that is introduced at the start of the disinfection process.
Chlorine
is added to achieve a higher level than is required at the end of the process.
This is to take account of the amount of chlorine that will be used to remove
all the bacteria & undesirable elements that are present in the water &
ensure that there will be enough chlorine residual left when the water is pumped
to the consumer.
The water can take several hours to clear the contact tank
this is important as it allows the disinfection process time to complete before
the water leaves the treatment works.
The second process control loop
that takes place is the addition of Sulphur dioxide or sodium bisulphate to
remove the excess chlorine from the water.
this final residual is calculated
to ensure residual is present as the water moves through any service reservoirs
& pipework on route to the customers tap.